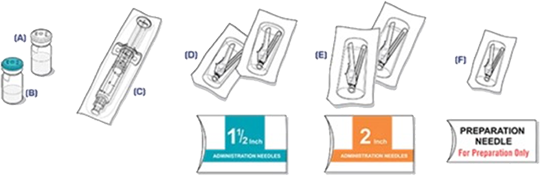
Storage of VIVITROL®1
The entire dose pack should be stored in the refrigerator (2 °C to 8 °C, 36 °F to 46 °F).
Unrefrigerated, VIVITROL can be stored at temperatures not exceeding 25 °C (77 °F) for no more than 7 days prior to administration. Do not expose the product to temperatures above 25 °C (77 °F). VIVITROL should not be frozen.
Keep out of reach of children.
VIVITROL must be prepared and administered by a healthcare provider.