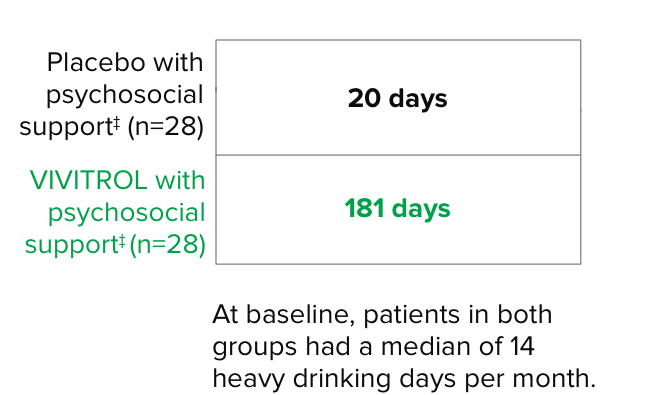
Primary endpoint2
The primary endpoint was the event rate of heavy drinking over the 24 weeks of treatment, defined as the number of heavy-drinking days divided by the number of days at risk for heavy drinking.2
Patients in the VIVITROL and counseling group had a greater reduction in days of heavy drinking than those in the placebo and counseling group.1,2
25%
Fewer heavy drinkinga
days per month
vs placebo2
VIVITROL 380 mg (n=205) vs placebo (n=209); HR=0.75 (0.60-0.94); P=0.02.
HR=hazard ratio.aHeavy drinking was defined as a self-report of ≥5 standard drinks consumed on a given day for male patients and ≥4 for female patients.
See study design.Please see study limitations.
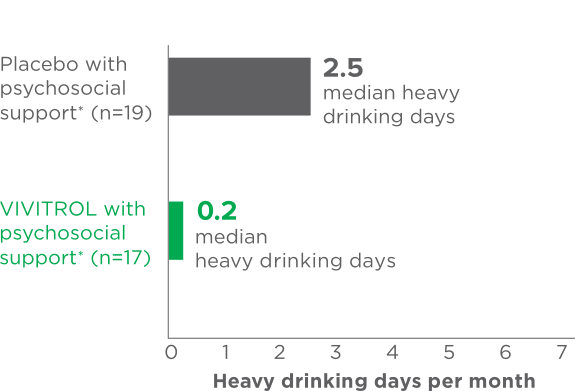
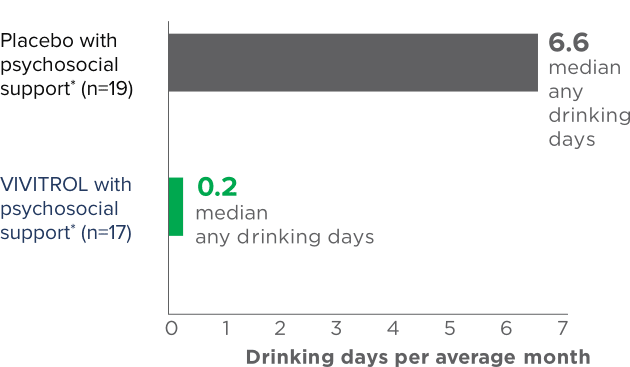
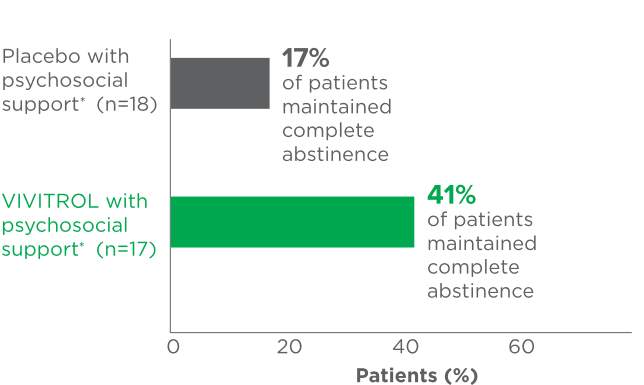
 fPsychosocial support was defined as biweekly counseling.
fPsychosocial support was defined as biweekly counseling.